Direct particle counting on a filter paper is a simple and rapid procedure where you either examine the filter directly with incident light or render it transparent so that you can apply transmitted light. The filter is placed directly on the movable stage of a binocular microscope with the contaminant side up. It is slowly traversed back and forth. As particles come into the field of view they are counted in several discrete size ranges.
Using the light microscope for direct counting on a filter offers a number of important advantages. You can:
• Determine the size distribution of particles.
• Detect large particles or fibers easily.
• Identify particles to locate sources of contamination.
You can vary the procedure to accomplish your specific goals. When you are only interested in very large particles (>150 µm), you can be less careful about cleaning your equipment. If appropriate, save time by counting particles down to 50 or 100 µm rather than down to 2 or 5 µm, since such procedures are adequate in many instances.
In all particle counting procedures, adequate illumination, well-aligned optics and careful operator training are necessary.
Sections:
• Filter Clearing
• Equipment
• Particle Counting
Filter Clearing
For transmitted light microscopy, you must render the filter transparent, a procedure called "clearing the filter." Several methods are available, but you should always use mixed esters of cellulose membrane filters. Jump to each filter clearing method:
• Acetone/Triacetin Method
• Dimethylphthalate and Diethyloxylate Method
• Microscope Immersion Oil Method
Acetone/Triacetin Method
1. Switch on the acetone vaporizer
2. Put a small volume of acetone in the syringe.
3. Cut the filter into four quarters using a rocking motion with a sharp scalpel.
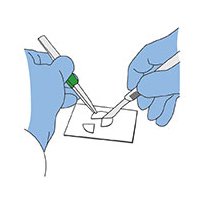
Cutting quarters from particle-laden 25 mm filter for subsequent clearing and microscopic examination.
4. Place a quarter of the membrane filter (sample side up) on a cleaned glass microscope slide. The other quarters are available for additional tests.
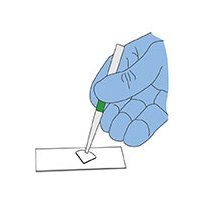
Placing filter section onto a cleaned 1" x 3" microscope slide.
5. Place the slide on the small stand approximately 2 cm below the outlet of the vaporizer.
6. Inject 0.25 mL of acetone. The filter normally clears immediately. If it does not totally clear, repeat the acetone injection and reduce the slide to outlet distance for subsequent filters.
7. Place one to three drops of glycerol triacetate (Triacetin) on the acetone-cleared filter.
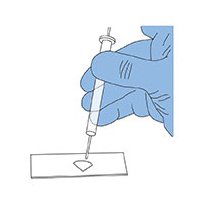
Adding Triacetin solution to acetone cleared filter.
8. Then immediately lower a clean cover slip onto the Triacetin at an angle. Do not press on the cover slip. A cover slip is essential if particles below 5 µm are to be counted.
9. Heat the filter for a few minutes to accelerate the clearing process (if needed). The mounted filter is stable and will not disintegrate.
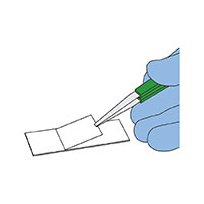
Cover slip placed at an angle over filter cleared using Triacetin.
Once the filter sample has been cleared and mounted, use phase contrast microscopy. If you used a gridded filter, the grids will remain visible to aid counting and to give a focal plane of reference.
Dimethylphthalate and Diethyloxylate Method
To prepare mounting medium:
1. Dissolve aerosol analysis filter in a k1:1 solution of dimethylphthalate and diethyloxylate (at a ratio of 0.2 g filter to 1 mL of solution). You can make up large volumes of this solution and store it out of sunlight in a stoppered bottle. Filter mounting medium as it is dispensed using a solvent-resistant syringe filter unit.
2. Place a drop of mounting medium on a freshly cleaned glass microscope slide to mount the membrane filter sample. For best results when cleaning slides, rinse with filtered CFC-Free Contact Cleaner.
3. Use a scalpel to cut a wedge-shaped piece from the filter with an arc length of about 1 cm. Carefully store the remaining filter. Avoid contamination in the event a second wedge must be cut.
4. Transfer the wedge of filter (keep sample side up) to the drop of mounting media using smooth tipped filter forceps. Cover with a cover slip. The filter becomes transparent in about 15 minutes at room temperature.
Microscope Immersion Oil Method
Using forceps, float the filter on a film of immersion oil in the cover of a plastic petri dish. Draw the filter over the rim of the cover to remove any excess oil and mount on the glass microscope slide.
Equipment
When using an Merck Fluid Contamination Analysis Kit to collect samples, you will need only the microscope illuminator, stage micrometer and tally counter.
A suitable microscope for particle counting should have:
• a binocular body
• a mechanical stage
• a multiple nosepiece
• 4X, 10X and 20X objectives
• a 10X Kellner or wide-field eye piece
Measuring Eyepiece (Reticle) Calibration
Before counting and measuring particles, you must calibrate the measuring eyepiece reticle of the microscope using a stage micrometer. Calibrate the scale with each objective to be used in the counting/measuring procedure.
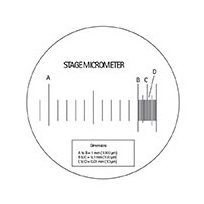
The stage micrometer is a glass slide with etched graduations.
A standard stage micrometer. (Click image to enlarge)
These graduations are accurately measured in millimeters as follows:
(a) From A to B = 1 mm (1000 µm); (b) From B to C = 0.1 mm (100 µm); (c) From C to D = 0.01 mm (10 µm).
1. Swing the lowest magnification objective into position.
2. Remove the eyepiece from the microscope to focus the eyepiece reticle. Look through the eyepiece with one eye and focus the reticle while keeping the second eye open and focused into the distance. This procedure minimizes eye strain when particle counting. Replace the eyepiece in the microscope.
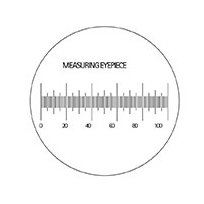
A standard measuring eyepiece (reticle) containing 100 linear graduations.
(Click image to enlarge.)
3. Place the stage micrometer onto the microscope stage. Adjust the microscope to bring the graduations of the stage micrometer into sharp focus.
4. Line up the eyepiece reticle with the stage micrometer.
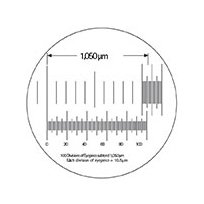
Based on the subdivisions of the stage micrometer (top), determine the scale of the divisions of the measuring eyepiece (bottom). This scale will remain constant at that magnification. (Click image to enlarge.)
Assuming that the example diagram represents what is seen when using a 4X objective (and 10X ocular), line up and calibrate the reticle divisions. Based upon 100 divisions of this reticle subtending 1050 µm on the stage micrometer, the calibration would be:
1050/100 = 10.5 μm per fine division
The figure of 10.5 µm/fine division would remain fixed for this particular combination of microscope, 4X objective, 10X eyepiece and reticle.
5. Repeat the above tests for the other objectives to be used.
6. Make a note of these calibration factors for future use with this microscope.
Particle Counting
Calibrate the eyepiece scale if this has not been done. When using transmitted light microscopy, you must first render the filter transparent. (See previous section "Filter Clearing.") This procedure results in a transparent wedge of filter mounted on a glass microscope slide. If you are using incident light microscopy, place the filter on a 2" x 3" glass microscope slide (1" x 3" is adequate for 25 mm filters). You may want to grease the slide lightly to hold the filter in place. A PetriSlide™ device may be used as an alternative.
More Instructions on Particle Counting
1. Mount the glass slide or PetriSlide™ device containing the filter onto the microscope stage.
2. Move the microscope stage so that the particles on the membrane appear to pass under the measuring eyepiece.
With a filter on the microscope stage, movement of the stage makes particles appear to pass under the divisions on the measuring eyepiece. (Click image to enlarge.)
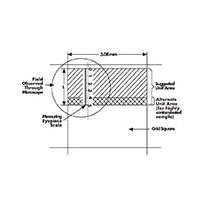
3. Count the number of particles in each designated size range found in a number of fields selected using the double diameter counting plan.
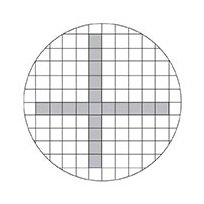
Double-diameter counting plan (count particles in shaded areas).
The number of particles counted multiplied by the number of fields should be equal to or greater than 500. A field may be any designated area, but is most commonly defined by the width of a grid square on the Merck filter (3.08 mm) and the length or a portion of the length of the measuring eyepiece scale. Always measure particle size by the longest dimension. Fibers (i.e. particles larger than 100 µm with a length to width ratio greater than 5:1) are usually listed separately.
4. Count the entire filter surface when counting a relatively small number of particles at low magnification.
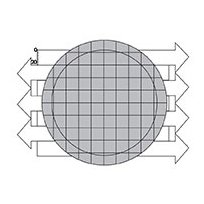
When scanning the entire filter at 40X magnification, the ocular micrometer scale is vertically aligned at the top left of the filtration area. The filter is scanned from left to right in the first pass, and each successive pass travels in the opposite direction.
When less than the entire filter surface is counted, multiply the number of particles actually counted by the total filter area divided by the area counted. The conversion factor to use is:
A/(3.08 LN)
Where:
A = Effective filtering area in mm² of the filter disc. For glass filter holders, the filtering area is 960 mm². Use 900 for field cassettes.
(For other filters and holders, refer to the website).
3.08 = Width in mm of filter grid square.
L = Length in mm of unit area.
N = Number of unit areas counted.
A typical counting worksheet is shown. Any particle size ranges may be used. These ranges are taken from SAE International's ARP-598A method, "The Determination of Particulate Contamination in Liquids by the Particle Count Method."
When you take samples of materials such as hydraulic fluids by means of the fluid sampler, it is important that you remove all excess fluid from the filter using a vacuum syringe before the cassette is opened. (See the "On-Line Sample Collection and Filtration" section in Chapter III for details on the fluid sampler.) Flushing solvent through the cassette at this point may seriously disturb the particle distribution.
It is always good practice to prepare a blank, proceeding through all the filtering and counting operations without introducing any sample to determine the "background" count. This is an excellent measure of glassware, solvent and technique cleanliness. Blank counts should not exceed 10% of the control limits established for the fluids being tested.
Image analysis systems and electronic counters that automate microscopic particle counting are now available and many component manufacturers are implementing them. The primary advantages of these systems are increased speed and the elimination of error due to operator fatigue. They do need careful calibration and careful filter preparation so that the particles lie in a single plane, as well as good contrast between particles and background.


















